Use Chemical Equation to Describe Double Decomposition Reaction
A reaction in which a single compound breaks into two or more simpler compounds is known as a decomposition reaction. Where two metal cations switch partners.
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single
2 NaCl s 2 Na s Cl 2 g Examples 2c-1 and 2c-2.

. If we had a container of hydrogen gas and burned this in the presence of oxygen the two gases would react together releasing energy to form water. Synthesis- two or more reactants unite to form a single product S Sulfur O2 Oxygen SO2 Sulphur dioxide Decomposition- A single reactant is decomposed or broken down into two or more CaCO3 Calcium Carbonate CaO Calcium oxide CO2 Carbon dioxide. 1 Zn 2 HCl 1 ZnCl 2 1 H 2 Single-Replacement.
Write a balanced chemical equation to describe this reaction. Decomposition of carbonates to yield oxides and carbon dioxide A general form for decomposition of metal carbonates is. NaCl s Na s Cl 2 g Step 3.
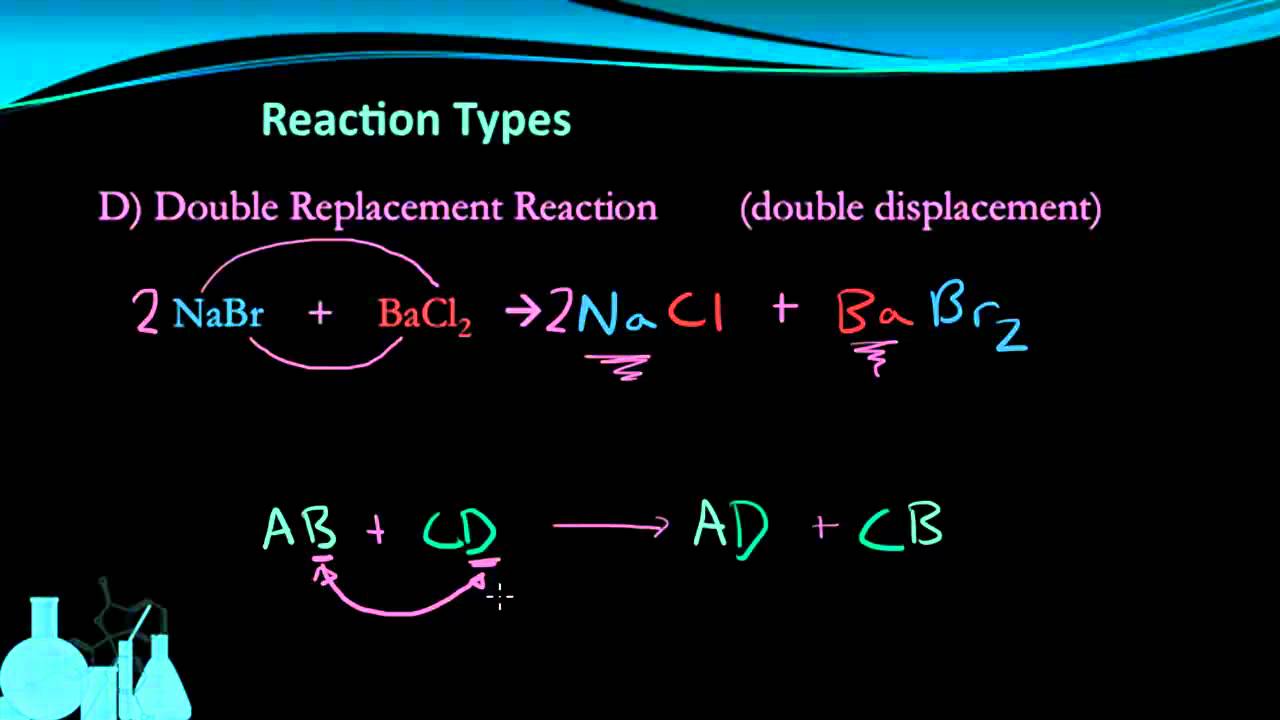
Predict the products of simple reactions given the reactants. The equation is often written in the general form AB CD CB AD The older term double decomposition has been largely replaced by the terms double displacement double replacement and metathesis. Instead the arrow is called a yield sign and so the equation is described as reactants yield products.
Include states of matter in your answer. To make things easy to understand Science. Decomposition Reactions 2 H 2O2 2 H 2O O 2 H O Decomposition reactions have the form A B C Single reactant breaks down into two or more products Chapter 5 Decomposition Reactions Further examples.
As it is reduced from 0 to -1 fluorine is the oxidizing agent. These reactions are in the general form. FeBr 3 aq 3NaOHaq FeOH 3 s 3NaBraq 17.
Smith Clark CC-BY-SA 40 GCC CHM 130 Chapter 10. Tap again to see term. 2 HgOs 2 Hgl O 2g This reaction was used by Joseph Priestley in the discovery of oxygen in 1774 CaCO 3s CaOs CO 2g.
Classify each reaction as a synthesis or combination decomposition single replacement double replacement or combustion reaction. Humans are surrounded by examples of chemical reaction. This is an example of a chemical change.
In every chemical reaction atoms are rearranged to give new substances. 2 Na 1 O 2 1 Na 2 O 2 Synthesis 3. When heated platinumIV chloride undergoes a decomposition reaction.
You can describe a chemical reaction by writing a word equation. Write a balanced chemical equation to describe this reaction. Just like following a recipe certain ingredients are.
Tap card to see definition. In a decomposition reaction de- off away -composition putting together arranging covalent bonds between components of a larger substrate molecule are broken down to form smaller product molecules. You just studied 53 terms.
Write the correct equation for the double replacement reaction between ironIII bromide and sodium hydroxide. Reaction Equation Type of Reaction 1. Write and balance the complete chemical equations to describe the process taking place be.
Be sure to do both of the following for reactions below. Use an activity series to predict whether a given reaction will occur and what the products will be. A double decomposition reaction is a type of decomposition reaction in which two constituent reactants interchange positive and negative ions and form two new compounds.
2 KI 1 PbNO 3 2 2 KNO 3 1 PbI 2 Double-Replacement 4. When silver metal is exposed to sulfur it reacts to form silver sulfide. 2 KClO 3 2 KCl 3 O 2 Decomposition 2.
H2 g F2 g 2HF g In the above-mentioned reaction the hydrogen is oxidized from 0 to 1 oxidation state and so this is the reducing agent. Lets take the reaction of hydrogen with oxygen to form water as an example. A D AD Redox reactions can be understood in terms of transfer of electrons from one involved species reducing agent to another oxidizing agent.
A change in which one or more substances are converted into new substances. Diphosphorus pentoxide water phosphoric acid. Classify reactions as synthesis decomposition single replacement double replacement or combustion.
It takes the form of XY X Y. Click card to see definition. When heated platinumIV chloride undergoes a decomposition reaction.
Therefore after this step the reaction is written as. Example of a decomposition reaction. Click again to see term.
- Chemical Equations o To be more precise we use chemical equations to describe a chemical reaction. 1 C 3 H 8 5 O 2 3 CO 2 4 H 2 O Heat Combustion 6. Unlike in a math equation a chemical equation does not use an equal sign.
Decomposition reactions release energy when covalent bonds in the substrate are broken down. Some chemical reactions are simple whereas others are quite complex However they can all be written by chemical equations that chemists use to describe chemical reactions. Synthesis decomposition single replacement and double replacement.
The burned matchstick turns to ashes and smoke while the iron forms a red patch over it. As with a word equation we use an arrow to indicate a reaction with the reactants on the left separated by a sign and products on the right also separated by. Consider the following chemical reaction written as a word equation.
In this process the former species is oxidized. A reaction between hydrogen gas and fluorine gas is the example of a redox reaction. This change is irreversible meaning that none of the previous substance can be brought back to its original form.
1 Cu 2 AgNO 3 1 CuNO 3 2 2 Ag Precipitation 5. The three primary types of decomposition reactions are. CaCO 3 CaO CO 2.
A decomposition reaction is often symbolized as AB. A decomposition reaction is just the opposite of combination reaction. A chemical equation is shorthand that scientists use to describe a chemical reaction.
Metal carbonates metal oxide carbon dioxide For example CaCO 3 s CaOs CO 2. Chemical Reactions YouTube Video on Single Replacement Reactions 1 Single Replacement Reaction 2 Single Replacement Reaction 3 108 Double Replacement Reactions M 1 X M 2 Y M 1 Y M 2 X Double Replacement Reaction. When lithium hydroxide pellets are added to a.
HClaq NaOHaq NaClaq HOHl Types of Decomposition Reaction. A double decomposition reaction is a reaction in which the positive ions and negative ions in two compounds switch partners to form two new compounds. Now up your study game with Learn mode.
Describe the importance of the mole ratio in stoichiometric.

Types Of Chemical Reactions Binary Decomposition Decomposition Of A Binary Compound Contains Exactl Chemistry Lessons Teaching Chemistry Chemistry Education

Chemistry Reactions Combination Reaction When Two Or More Reactions Combine To Form Chemical Reactions Chemical Fun Science

Predicting Products For Decomposition Reactions Youtube Reactions Mo Co Chemistry
Comments
Post a Comment